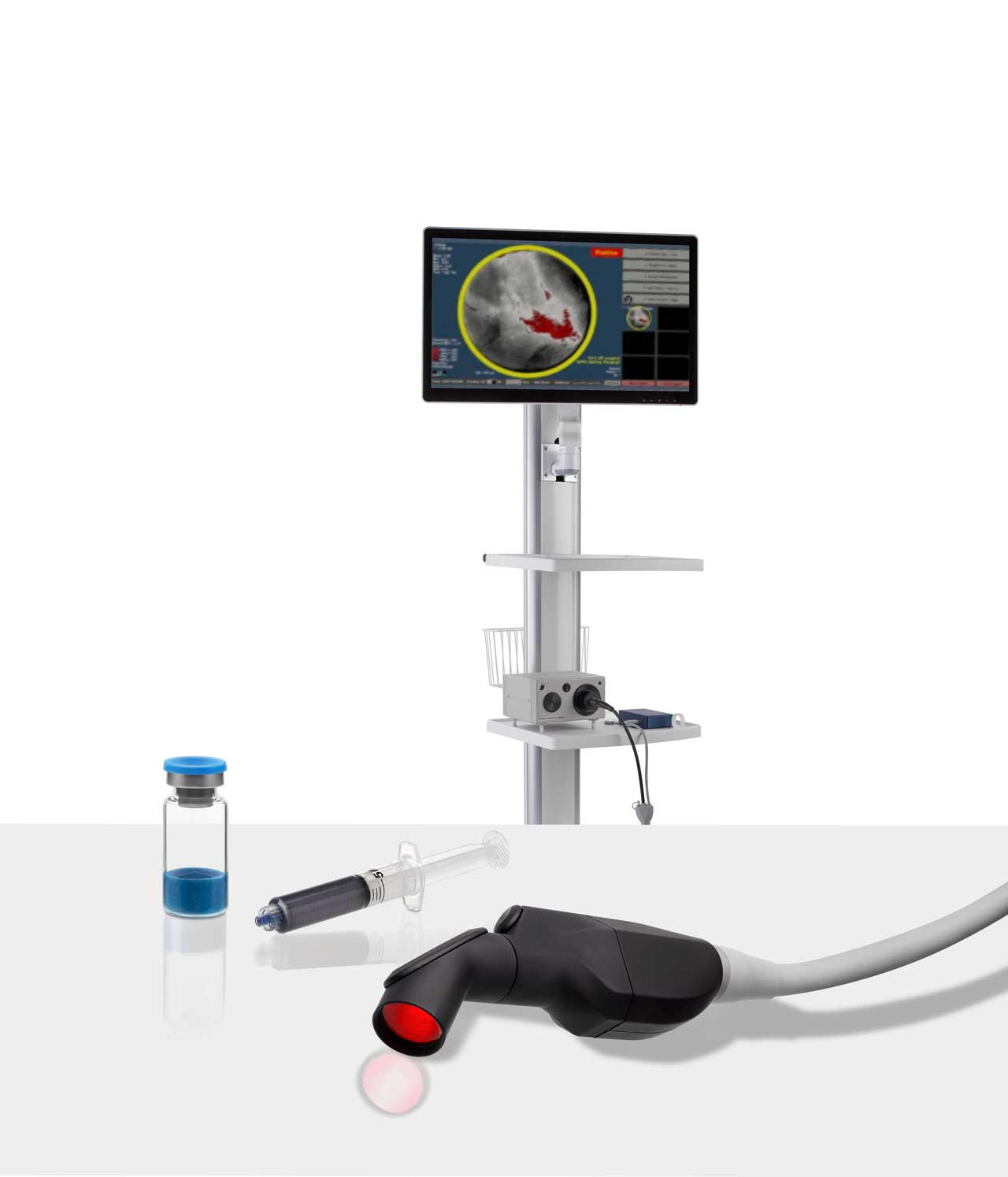
The Science Propelling Lumicell
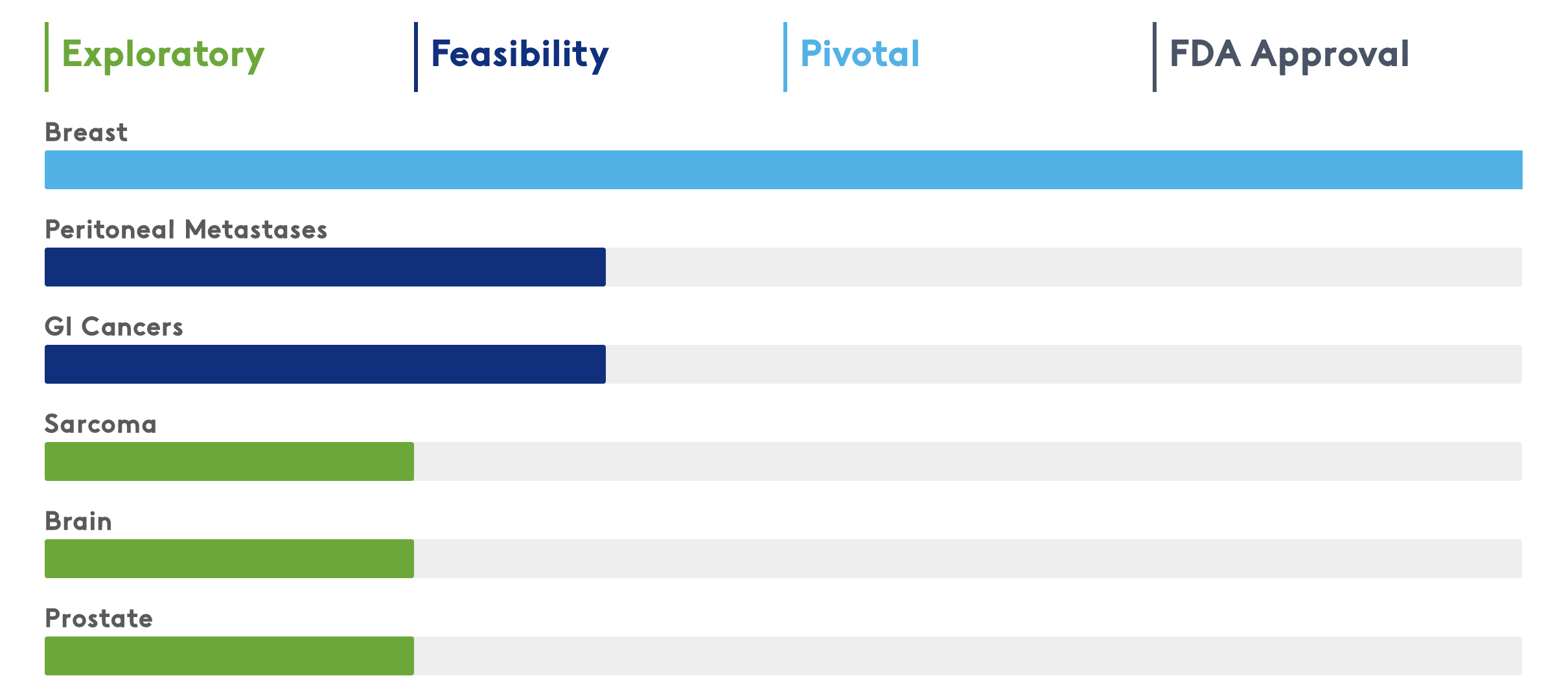
Clinical Trials in Multiple Solid Tumor Indications
LUMISIGHT™ is a prodrug that is designed to be optically inactive when intact and produces a fluorescent signal after its peptide chain is cleaved by cathepsins and matrix metalloproteases (MMPs). The levels of these enzymes are higher in and around tumor and tumor-associated cells than in normal cells.
The Lumicell™ Direct Visualization System (DVS) has potential utility for development in multiple solid tumor indications. Over 800 patients have been clinically evaluated in 7 different cancer types across 18 leading medical centers in the United States.
Click to expand graphic.
Awarded Breakthrough Device Designation for the Lumicell DVS in breast, GI and all solid tumors. Awarded Fast Track Designation for the LUMISIGHT Optical Imaging Agent for breast cancer.
![]()
![]()
"I am passionate about helping improve cancer outcomes."
Dan Harris

Completed Clinical Trials
Breast Cancer
February 2024
Feasibility study (NCT04440982), to evaluate for the first-time use of the Lumicell DVS in breast cancer patients undergoing neoadjuvant therapy
May 2022
Lumicell’s pivotal INSITE (Investigation of Novel Surgical Imaging for Tumor Excision) Breast Cancer clinical trial (NCT03686215). Results of the INSITE trial are published in NEJM Evidence and have been included in Lumicell’s Premarket Approval and New Drug Application submissions to the U.S. Food & Drug Administration
April 2020
Multi-Center feasibility study in breast cancer
January 2016
Phase 1 safety study in breast cancer and sarcoma
Duke University Medical Center
Prostate Cancer (ex vivo)
February 2021
Feasibility study to detect prostate cancer in resected tissue; next steps include an in vivo study (NCT03441464)
Ongoing Clinical Trials
Gastrointestinal Cancer (ex vivo)
Initiated August 2016
Feasibility study of the Lumicell DVS to detect GI cancers including gastric, colorectal, pancreatic, and esophageal cancers (NCT03834272)
Peritoneal Metastases (in vivo)
Initiated March 2019
Feasibility study of the Lumicell DVS to detect peritoneal surface malignancies, including peritoneal metastases from primary ovarian and colon cancer, as well as mesothelioma (NCT03834272)
Brain Cancer (in vivo)
Initiated May 2019
Feasibility study of the Lumicell DVS to detect brain cancers that include glioblastomas, low-grade gliomas and metastases to the brain (NCT03717142)
For detailed information on Lumicell’s clinical trials, visit ClinicalTrials.gov.
Lumicell In Key Peer-Reviewed Publications
- Intraoperative fluorescence guidance for breast cancer lumpectomy surgery (2023)
- Clinical impact of intraoperative margin assessment in breast-conserving surgery with a novel pegulicianine fluorescence-guided system: a nonrandomized controlled trial (2022)
- Performance of a novel protease-activated fluorescent imaging system for intraoperative detection of residual breast cancer during breast conserving surgery (2021)
- Feasibility study of a novel protease-activated fluorescent imaging system for real-time, intraoperative detection of residual breast cancer in breast conserving surgery (2020)
- Real-time, intraoperative detection of residual breast cancer in lumpectomy cavity walls using a novel cathepsin-activated fluorescent imaging system (2018)
- A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer (2016)